Posted by Dan | Filed under earth, water, air, weather, education, skills, news, information, Immaterial Things, life, energy, resources, Material Things, Natural
Seemed to me like a question long answered. This is my shortened answer:
Heat is a macroscopic characteristic of ‘microscopic’ events. In our case we have 2 bodies of water with different temperatures. The higher the temperature the higher the kinetic energy of the particles.
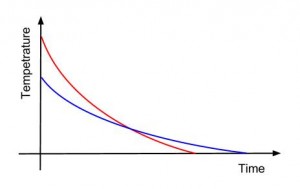
It is a fact that hot water freeze faster than cold water, and we have made this simple chart to represent this fact.
We can see that the hot water looses temperature faster than the cooler one and at one point their temperatures will be the same. Yet at that point the inertia of it’s particles kinetic movement would would be higher than the initially cooler water mass and because of that the hotter water will continue to loose temperature faster. This is the critical factor that determines the fact that the hot water body freeze faster. We refer here to thermal conduction witch is the principal (quantitative) means of heat transfer in the case of water bodies. We can easily see that radiation, net mass transfer, only have a very small impact on the water heat transfer while viscosity (booth bodies have the same viscosity) and chemical disipation and not specific to the present question.
So the final answer is: Because of the intertia of the kinetic movement of particles.
You believe this is wrong ? Thanks for leaving a comment.
Tags: cold water, freeze, hot water, Q&A
Permalink |
|
June 28, 2012
Leave a Reply
